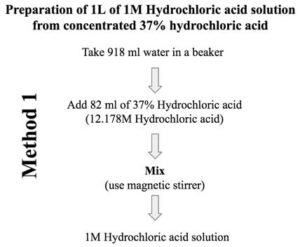
What volume of 12.5 M concentrated HCl is required to make 1 L of 0.1 M HCl solution?A. 7 mLB. 9 mLC. 8 mLD. 10 mL

SOLVED: Solution Preparation and Standardization Name: Hydriodic Acid Calculations Calculate the volume of concentrated (12 M) HCI required to make 100.0 mL of a 1M solution: CcVr = CaVd (12 M)(Vr) = (

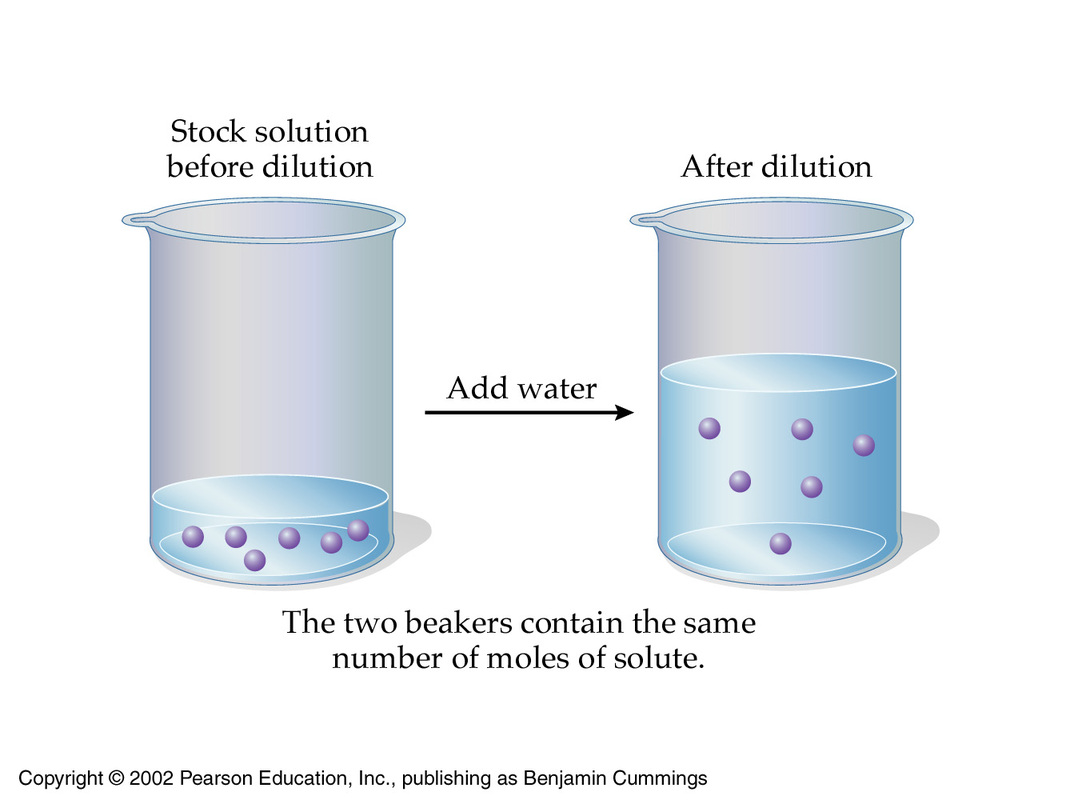
SOLVED: I would dilute 100.0 mL of a 5.0 M HCl solution to make a 0.20 M HCl solution. What will be the final volume? (2 pts) 10. Calculate the molarity of
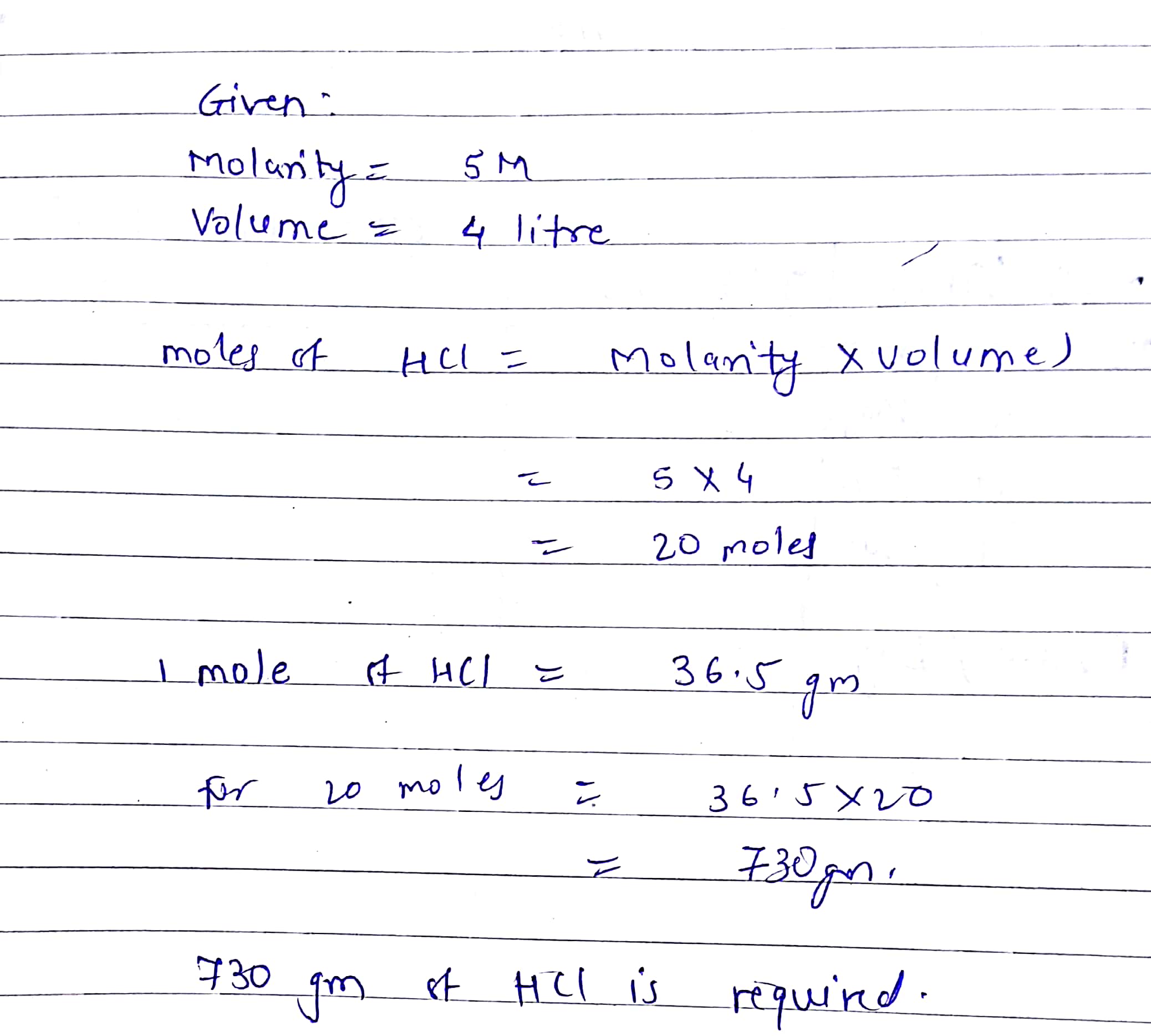
How many grams of hydrogen chloride,HCL are required to prepare 4 Litre of 5M HCL in water - 2ilx7jll
If 250ml of 1M HCl is diluted to 1000ml, what would be molarity of the diluted solution? What will be the pH? - Quora

Discussion | 31 5. How many grams are needed to make 2.5L of 1.5M HCl solution? CI 350 レ. 6. How many grams are needed to make 500...

A 37%(w/w) solution of Hydrochloric acid has a density of 1 18 g/mol What volume of this solution should - Chemistry - Some Basic Concepts of Chemistry - 13482901 | Meritnation.com

1. Which Vhich will basic buffer? 100 mL of 0.1 M HCl + 100 ml of 0 1 (a) 100 mL of o M NaOH (b) 50 mL of 0.1 M M







![BT021] 1M Tris-HCl, pH 8.5 | Biosolution BT021] 1M Tris-HCl, pH 8.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/05/BT016-1M-Tris-HCl.jpg)